Asymmetric total synthesis of nodulisporiviridin E†
Abstract
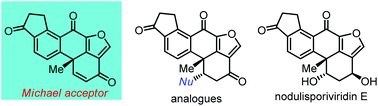
The asymmetric total synthesis of (+)-nodulisporiviridin E, a fungal metabolite from Nodulisporium sp., was achieved in 16 steps. The synthesis features a convergent approach from two conventional fragments. An intramolecular Heck cyclization was applied to construct the C-ring and the all-carbon quaternary center at C-10. The furan E ring was installed by means of an intramolecular oxonium trapping reaction. This approach provides an advanced Michael acceptor, which might facilitate the preparation of various analogues and derivatives for biological studies.


 Please wait while we load your content...
Please wait while we load your content...