Metal-free insertion of sulfur dioxide with aryl iodides under ultraviolet irradiation: direct access to sulfonated cyclic compounds†
Abstract
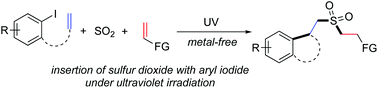
Metal-free insertion of sulfur dioxide with aryl iodides and silyl enolates or allylic bromide under ultraviolet irradiation at room temperature is accomplished. This protocol provides a convenient route to sulfonated cyclic compounds under mild conditions. Not only N-(2-iodophenyl)-N-methylmethacrylamides but also 1-iodo-2-allenoxybenzene is workable. A plausible mechanism is proposed, which shows that during the reaction process, aryl radicals formed in situ from aryl iodides under ultraviolet irradiation undergo intramolecular 5-exo-cyclization, with subsequent sulfonylation via insertion of sulfur dioxide. The resulting sulfonyl radicals are further trapped by silyl enolates or allylic bromide giving rise to sulfonated cyclic compounds.


 Please wait while we load your content...
Please wait while we load your content...