An asymmetric multicatalytic reaction sequence of 2-hydroxycinnamaldehydes and enolic 1,3-dicarbonyl compounds to construct bridged bicyclic acetals†
Abstract
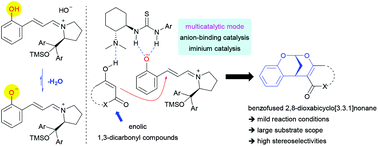
An efficient asymmetric multicatalytic system involving iminium catalysis and anion-binding catalysis has been developed, which exhibited high catalytic activity in a one-pot, two-step reaction sequence of 2-hydroxycinnamaldehydes and enolic 1,3-dicarbonyl nucleophiles to construct an enantioenriched 2,8-dioxabicyclo[3.3.1]nonane scaffold. The inherent phenolic hydroxyl group of 2-hydroxycinnamaldehyde played an important role in the catalytic process, which potentially provided a phenoxide anion to combine with the thiourea moiety of a bifunctional tertiary amine-thiourea catalyst via anion binding, thus making the developed multicatalytic system more economic and different from the previously reported catalytic mode.


 Please wait while we load your content...
Please wait while we load your content...