Ag-Catalyzed cycloisomerization of 1,6-enynamide: an intramolecular type II Alder-ene reaction†
Abstract
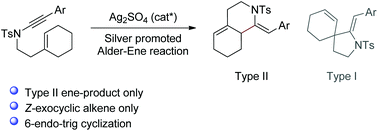
Promoted by a silver catalyst, 1,6-enynamides with a cyclic ene moiety can undergo cycloisomerization to fused N-heterobicycles. This transformation presents an unusual type II Alder-ene reaction and is believed to proceed through a catalytic cycle of formation of a keteneiminium silver intermediate, subsequent 6-endo-trig cyclization and protodemetalation.


 Please wait while we load your content...
Please wait while we load your content...