Spiroalanpyrroids A and B, sesquiterpene alkaloids with a unique spiro-eudesmanolide–pyrrolizidine skeleton from Inula helenium†
Abstract
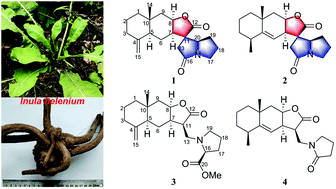
Spiroalanpyrroids A (1) and B (2), two sesquiterpene alkaloids with an unprecedented eudesmanolide–pyrrolizidine spiro[5.5] framework, were isolated together with two new sesquiterpene-amino acid adducts, helenalanprolines A (3) and B (4), from the roots of Inula helenium. Their structures were elucidated by spectroscopic data analysis, ECD calculations, and single-crystal X-ray diffraction analysis. A plausible biosynthetic pathway was proposed for 1 and 2. Bioassays showed that compounds 3 and 4 significantly inhibited nitric oxide production in lipopolysaccharide-induced RAW264.7 macrophages with IC50 values of 15.8 and 13.5 μM, respectively.


 Please wait while we load your content...
Please wait while we load your content...