Cyclamenols E and F, two diastereoisomeric bicyclic macrolactams with a cyclopentane moiety from an Antarctic Streptomyces species†
Abstract
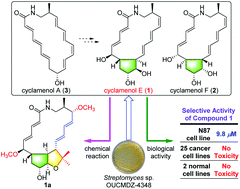
Cyclamenols E (1) and F (2), two bicyclic macrolactams possessing a cyclopentane moiety, were isolated from the Antarctic Streptomyces sp. OUCMDZ-4348. Their structures, including absolute configurations, were determined by spectroscopic analysis, chemical derivatization, and ECD calculation. Treatment of compound 1 with 2,2-dimethoxypropane and pyridinium p-toluenesulfonate in acetone/DMF led to the formation of acetonide 1a with an unexpected skeleton. Compound 1 showed moderate inhibitory activity against the gastric carcinoma cell line N87 with an IC50 value of 9.8 μM, but no cytotoxicity was observed on the other 25 cancer cell lines tested.


 Please wait while we load your content...
Please wait while we load your content...