The enantioselective construction of trifluoromethylated quaternary stereocenters via the Rh-catalyzed asymmetric dehydrated arylation of unprotected hemiaminals†
Abstract
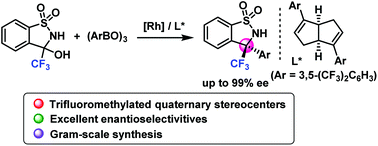
A method for the highly enantioselective Rh-catalyzed dehydrated arylation of unprotected hemiaminals has been developed, and provided a series of chiral benzosultams bearing trifluoromethylated quaternary stereocenters in high yields with excellent enantioselectivities (up to 99% ee). The synthetic utility was further demonstrated by the facile enantioselective construction of a HIV-1 inhibitor molecule.


 Please wait while we load your content...
Please wait while we load your content...