One-pot generation of benzynes from 2-aminophenylboronates via a Rh(ii)-catalyzed N–H amination/oxidation/elimination cascade process†
Abstract
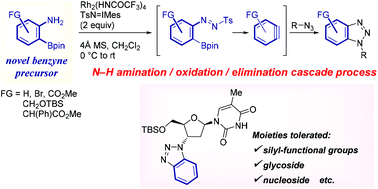
This article describes the first application of 2-aminophenylboronates as precursors for benzynes. Utilizing Rh2(HNCOCF3)4 as the catalyst, Rh(II)-nitrene-mediated N–H amination of the starting material triggered a cascade of oxidation/elimination processes resulting in the generation of benzynes, thus providing suitable conditions for a one-pot cycloaddition with azides or furans. The transformation proceeded under acid-, base-, and fluoride-free conditions, below ambient temperature, and was applicable to a range of substrates containing glycoside and nucleoside moieties, as well as silyl-functional groups.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...