Allylation of β-amino phosphonic acid precursor via palladium-NHC catalyzed allylic C–H activation†
Abstract
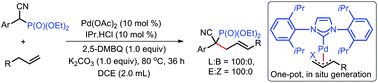
Allylic C–H alkylation of allylbenzene with α-cyano-phosphate ester has been achieved via Pd(II)/N-heterocyclic carbene (NHC) catalysis under mild reaction conditions and provides the corresponding linear allylation products in moderate to good yields. This reaction establishes a new method to construct β-amino phosphonic acid derivatives with tertiary phosphonates.


 Please wait while we load your content...
Please wait while we load your content...