Transition-metal-free direct C-3 cyanation of quinoxalin-2(1H)-ones with ammonium thiocyanate as the “CN” source†
Abstract
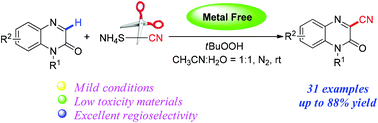
A novel avenue of C–CN bond construction was facilely achieved via the TBHP-mediated oxidative coupling reaction between quinoxalin-2(1H)-one derivatives and ammonium thiocyanate without any metal catalyst. Applying this protocol, a variety of 3-cyanoquinoxalin-2(1H)-one derivatives were prepared in moderate to good yields with good functional-group tolerance under mild conditions. Additionally, this methodology featured a broad substrate scope, excellent regioselectivity and operational simplicity.


 Please wait while we load your content...
Please wait while we load your content...