Enantioselective synthesis of chiral multicyclic γ-lactones via dynamic kinetic resolution of racemic γ-keto carboxylic acids†
Abstract
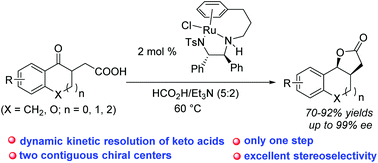
Ru-Catalyzed asymmetric transfer hydrogenation of γ-keto carboxylic acids has been achieved by using the formic acid–triethylamine azeotrope as the hydrogen source, affording chiral multicyclic γ-lactones in high yields with excellent diastereo- and enantioselectivities (up to 92% yield, >20 : 1 dr and 99% ee). This method provides a highly efficient approach to obtain valuable multicyclic γ-lactones through a reduction/lactonization sequence. Moreover, a concise synthetic route to obtain bioactive molecules (+)-GR24 and (+)-epi-GR24 has also been developed by using this methodology as a key step.


 Please wait while we load your content...
Please wait while we load your content...