Total syntheses of (−)-15-oxopuupehenol and (+)-puupehenone and formal syntheses of (−)-puupehenol and (+)-puupehedione†
Abstract
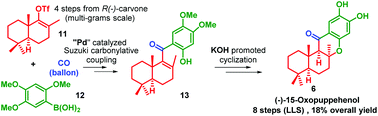
A concise total synthesis of antitumor and antimalarial marine sponge metabolite (−)-15-oxopuupehenol has been accomplished in 8 steps (longest linear route) with an overall yield of 18% from R-(−)-carvone. Our synthesis involves a Suzuki carbonylative coupling reaction to assemble the compact tetra-substituted α,β-unsaturated aryl ketone and a KOH promoted intramolecular cyclization reaction to construct the unique chromanone core. The antituberculosis drug (+)-puupehenone is obtained via a three-step transformation from the synthetic (−)-15-oxopuupehenol. The formal syntheses of (−)-puupehenol and (+)-puupehedione can also be achieved from the same advanced intermediate 19.


 Please wait while we load your content...
Please wait while we load your content...