Zinc(ii)-mediated diastereoselective Passerini reactions of biocatalytically desymmetrised renewable inputs†
Abstract
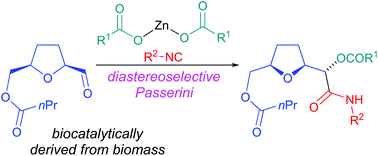
2,5-Bis(hydroxymethyl)tetrahydrofuran, a renewable building block, has been efficiently desymmetrised to give either enantiomers of the corresponding monobutyrate in high ee exploiting a biocatalytic methodology. The aldehydes derived from these monoesters have been subjected to a series of diastereoselective Passerini reactions. Careful optimization of reaction conditions has allowed for the increase of the dr from 1.5 : 1 to 9 : 1. The best results were obtained with zinc(II)-mediated reactions. In particular, a new modification of Passerini reaction that employs zinc dicarboxylates has been introduced.


 Please wait while we load your content...
Please wait while we load your content...