Nanocapsules with stimuli-responsive moieties for controlled release employing light and enzymatic triggers†
Abstract
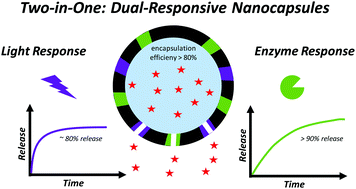
The development of stimuli-responsive nanomaterials, that possess tailored functional properties for the release of specific compounds, is of particular interest. To this extent, controlling the release of molecules at the desired target is an important parameter to regulate chemical and/or biological reactions at a more profound level in a wide variety of applications. In the present work, we report on the development of dual-responsive thiourethane–urethane nanocapsules synthesized via an interfacial polymerization reaction executed at the droplet interface using the inverse miniemulsion technique. Evidence via morphological and controlled release investigations indicate that our nanocapsules are able to encapsulate hydrophilic compounds with high efficiency in their aqueous core and allow for its selective release upon exposure to UV light and the enzyme esterase. Moreover, we demonstrate the efficient encapsulation of the fragrance molecule geranyl acetate and the anticancer drug doxorubicin. For the latter, we demonstrate its apoptotic effect after being released in MCF 7 breast cancer cells. Overall, these nanocapsules can be used for a wide variety of applications where a selective release of the payload is desired.


 Please wait while we load your content...
Please wait while we load your content...