Conjugated molecules for colourimetric and fluorimetric sensing of sodium and potassium†
Abstract
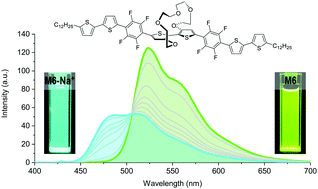
We present a series of conjugated molecules for colourimetric and fluorimetric sensing of alkali metal cations Na+ and K+. The dye molecules are based on an electron-rich bithiophene core bridged by an oligoethylene glycol bridge capable of selectively binding either sodium or potassium. The molecules are further functionalised with electron-rich or electron-deficient moieties to modulate the band gap and thus shift the absorbance across the visible range. We observe distinct colour changes and high selectivity for alkali metal cations at millimolar concentrations. We moreover see highly selective fluorimetric responses with the molecules exhibiting either turn-on, turn-off or ratiometric sensing behaviour. We finally demonstrate a dual optical sensor capable of distinguishing between sodium and potassium in solution.
- This article is part of the themed collections: Recent Open Access Articles in Frontiers Journals and Emerging Organic Electronics


 Please wait while we load your content...
Please wait while we load your content...