Each phenyl group performs its own functions on luminescence: phenyl substituted effect in tetraphenylpyrazine†
Abstract
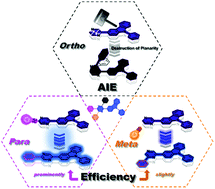
Aggregation-induced emission (AIE) has drawn considerable attention owing to its interesting phenomenon, and the AIE mechanisms of different molecule systems have been gradually revealed. Here, we investigated the difference in 3-carbazole-pyrazine-based isomers and explored the effects of three substituted phenyl groups (ortho, meta and para) on the tetraphenylpyrazine derivatives (TPPs). The meta- and para-phenyl groups could slightly and significantly adjust their emission properties, respectively. The ortho-phenyl group could distort the pyrazine plane owing to the large steric hinderance of the two moieties; this triggered many molecular motions and led to the wastage of excited state energy, stimulating the AIE characteristics of TPPs. This result is demonstrated for other pyrazine derivatives and can serve as a design strategy of AIE molecules.
- This article is part of the themed collection: Recent Progress on Aggregation-Induced Emission


 Please wait while we load your content...
Please wait while we load your content...