The first connection of carbonyl-bridged triarylamine and diketopyrrolopyrrole functionalities to generate a three-dimensional, non-fullerene electron acceptor†
Abstract
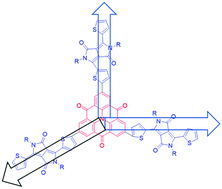
We report for the first time the use of a carbonyl-bridged triarylamine core with diketopyrrolopyrrole terminal units to generate a three-dimensional, non-planar non-fullerene electron acceptor with favourable properties for use in organic photovoltaic devices. The carbonyl-bridged triarylamine-functionalized, small molecule non-fullerene electron acceptor, 2,6,10-tris(5-(2,5-bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)-4H-benzo[9,1]quinolizino[3,4,5,6,7-defg]acridine-4,8,12-trione (coded as R1), was synthesized via the industrially viable, Suzuki cross-coupling reaction using commercially and cheaply available substrates. Using PTB7 as a donor, a power conversion efficiency of 9.33% was achieved in simple, solution-processable bulk-heterojunction devices, a result that is amongst the best in the literature for three-dimensional non-fullerene acceptors.


 Please wait while we load your content...
Please wait while we load your content...