Nitro-graphene oxide in iridium oxide hybrids: electrochemical modulation of N-graphene redox states and charge capacities†
Abstract
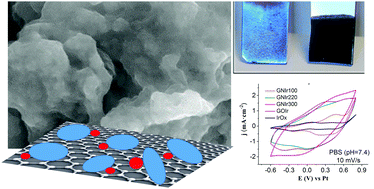
Carbon plays a significant role in the development of electrode materials used in, for example, catalysis, energy storage and sensing. Graphene-based coatings and carbon nanotubes have expanded that role through nanostructuring of hybrids or the formation of composites. In particular, the formation of hybrids of nanocarbons with iridium oxide yield nanostructured materials through direct anodic deposition, which have substantially improved charge capacities vs. pure IrOx. Modification of the possible redox sites, new structuring of the hybrids and increased charge capacities are expected as a result. This work shows that nitrogen (N)-doped graphenes, as part of an IrOx hybrid, offer a new redox chemistry on graphene oxide through electrochemical modulation of the redox states of nitrogen in graphene, and yield stable nitro groups bound to carbon, which have, so far, the largest oxidation state reported in N-doped graphene. The hybrid materials are obtained in the form of coatings thanks to spontaneous adhesion of iridium oxo species on N-doped graphenes and further anodic electrodeposition of the mixture. While the oxidizing synthesis process already involves modification of the oxidation state of nitrogen, further electrochemical cycling evidences the electrochemical processes for both the IrOx and N groups attached to the graphene oxide. All the hybrids obtained present a wide range of nitrogen-based groups that include the nitro group, and a significant charge capacity that remains large upon electrochemical cycling and that involves all the faradaic processes from the iridium and graphene components. One hybrid, in particular, which includes the highest starting oxidation state, reaches a significantly higher charge capacity, higher even than the graphene oxide hybrid, and with 70% retention upon cycling. Although nitrogen doping of graphene is considered to be a reducing process, this study shows that an oxidizing range of nitrogen doping is also possible. IrOx, and the reversible redox processes that iridium offers, are thought to be essential in stabilizing an unusual nitro-carbon-oxide system and allowing a sustained high charge storage capacity that is twice that of pristine graphene or graphene oxide hybrids.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...