Facile synthesis of nickel–copper hollow spheres as efficient bifunctional electrocatalysts for overall water splitting†
Abstract
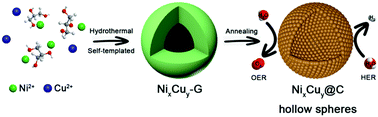
Sustainable energy conversion has motivated researchers to design and develop novel efficient bifunctional electrocatalysts for water splitting. In this work, we report the facile synthesis of NixCuy@C hollow spheres by using a template-free strategy. The activities of the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) are significantly enhanced by the hollow structure and optimal composition. The as-synthesized Ni4Cu2@C hollow spheres exhibit superior HER performance with overpotentials of 55 and 91 mV at 10 mA cm−2 in alkaline and acidic media, and the low overpotential of 280 mV at 10 mA cm−2 for the OER in alkaline media. When Ni4Cu2@C hollow spheres are employed as electrocatalysts for both the anode and cathode in an overall water splitting system, a cell voltage of only 1.49 V at 15 mA cm−2 and long-term durability (50 h@40 mA cm−2) are achieved.


 Please wait while we load your content...
Please wait while we load your content...