Phenazine-based photosensitizers for singlet oxygen generation†
Abstract
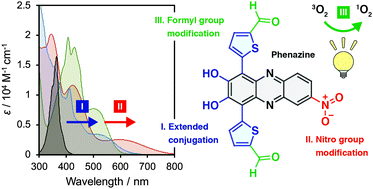
Photosensitizers with the ability to produce singlet oxygen (1O2) under light irradiation have attracted much attention because of their fascinating applications as represented by photodynamic therapy. A family of heteroanthracenes have been extensively studied as photosensitizers because of their high quantum yields of 1O2 generation as well as tetrapyrroles. However, the previous research studies lack insight into phenazine-based photosensitizers despite their considerable potential. Here we develop new phenazine derivatives and investigate their photophysical properties and photosensitizing abilities to produce 1O2. The photoabsorption of phenazine is enhanced in the long wavelength region by the introduction of two hydroxyl groups and conjugation with two thiophene rings, and further by modification with a nitro group, which extends the photoabsorption to the near-infrared region but interferes with the photosensitized 1O2 generation due to the fast nonradiative decay of excited states. A superior photosensitizing ability to produce 1O2 is imparted to the new phenazines by modification with formyl groups, according to El-Sayed's rule. The phenazine-based photosensitizers with improved photoabsorption and 1O2 quantum yields promise future practical applications, and the obtained findings will contribute to the establishment of synthetic guidelines for new photosensitizers with the excellent ability to produce 1O2.


 Please wait while we load your content...
Please wait while we load your content...