A zinc-responsive fluorophore based on 5′-(p-hydroxyphenyl)-pyridylthiazole†
Abstract
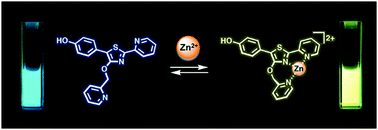
The synthesis and photophysical properties of an ion-sensitive fluorescent compound are described, featuring 5′-(p-hydroxyphenyl)pyridylthiazole (HPPT) as a highly emissive fluorophore. Density functional theory (DFT) calculations indicate that HPPT is capable of intramolecular charge transfer (ICT), with further polarization upon complexation with Zn(II). A 4′-picolyloxy–HPPT derivative was prepared and determined to form a 1 : 1 complex with Zn(NO3)2 in acetonitrile, with a Stokes shift of 137 nm (6323 cm−1) and a 67 nm bathochromic shift in emission relative to the neutral ligand, and a fluorescence quantum yield (ΦPL) of 92%. An X-ray crystal structure of the HPPT–Zn(II) complex confirmed a tridentate structure with a seven-membered chelate ring, and the picolyloxy unit rotated out of plane.
- This article is part of the themed collection: Celebrating Jean-Marie Lehn’s 80th Birthday


 Please wait while we load your content...
Please wait while we load your content...
