A computational study of the reactivity of rare-earth/phosphorus Lewis pairs toward polymerization of conjugated polar alkenes†
Abstract
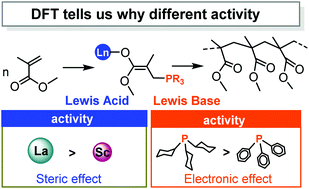
The polymerization mechanism of methyl methacrylate (MMA) catalyzed by rare-earth/phosphorus (RE/P) Lewis pairs has been systematically studied through density functional theory (DFT) calculations. Having achieved an agreement between theory and experiment, it is found that the polymerization of MMA mediated by intermolecular RE/P Lewis pairs mainly follows the bimetallic mechanism, while the monometallic pathway could not be excluded in the case of a La analogue. In comparison with phenyl phosphorus as a Lewis base, the higher activity of cyclohexyl phosphorus toward MMA polymerization could be ascribed to the electron-donation ability, rendering more electron flow in the addition reaction. Besides, a computational modelling of analogous intramolecular RE/P systems indicates that the size of the central metal and the length of the chain connecting Lewis pairs play an important role in the catalytic activity.
- This article is part of the themed collection: Rare Earth Chemistry – In memory of Professor Xu Guangxian at his centenary


 Please wait while we load your content...
Please wait while we load your content...