Stabilization of polyiodide networks with Cu(ii) complexes of small methylated polyazacyclophanes: shifting directional control from H-bonds to I⋯I interactions†
Abstract
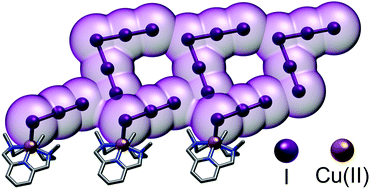
Ordered polyiodide networks have recently gathered considerable attention as electronic materials, a topic historically dominated by metals. Could we incorporate metal cations into polyiodide frameworks in a controlled manner to simultaneously boost electronic properties and robustness of these materials? Herein we present a first principles study featuring three analogous polyazacyclophanes (L, L-Me, L-Me3), differing only in the extent of N-methylation. We demonstrate (potentiometry, ITC) how they all form the same CuL2+ (L = L, L-Me, L-Me3) complex as prevalent species in solution, so that a level playing field exists where only N-methylation distinguishes them. Then we use them as countercations for polyiodide growth. XRD analysis of the resulting crystals clearly shows that methylation is a valuable tool to gradually shift directional control of subtending pairing preferences from H-bond to I⋯I interactions: this affects global packing and actively incorporates metal centres into polyiodide chains, setting the scene for further developments.


 Please wait while we load your content...
Please wait while we load your content...