Tailoring the structures and transformations between copper complexes in gas–solid reactions and solid-state synthesis†
Abstract
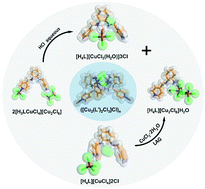
In this study, two copper(II) salts with different configurations, 2[H3LCuCl4][Cu2Cl6] (1) and [H4L][CuCl4]2Cl (2) (L = (1R,2R)-N,N′-bis(pyridin-4-ylmethyl)cyclohexane-1,2-diamine), have been synthesized and applied in the following solid–gas and solid–solid reactions. Both salts 1 and 2 gave rise to the salt ([H4L][Cu2Cl8]H2O (3) upon exposure to HCl gas or grinding with equal moles of CuCl2·2H2O. In the reaction from 1 to 3, the Cu–N coordination bond of [H3LCuCl4]+ in 1 was broken by the hydrated vapours of HCl and new N–H, Cu–Cl–Cu and Cu-H2O bonds of mixture products 3 and [H4L][CuCl3(H2O)]3Cl (4) were generated. In the reaction from 2 to 3, a stoichiometric ratio-controlled transformation occurred with the change in the geometry of copper(II) anions from [CuCl4]2− to [Cu2Cl8]4−. The detailed structural changes between covalent and coordination bonds and the transformations of these copper complexes are described in the paper, which has provided a good model for understanding the reaction pathways and insight into the structural transformation of materials of interest. Recrystallization of the grinding product of the ligand L with CuCl2·2H2O obtained a novel coordination polymer ([Cu2(L′)2Cl3]Cl)n (5, L′ = (1R,2R)-N-(pyridin-4-ylmethyl)cyclohexane-1,2-diamine) with a clear channel of the pore size being 16 × 13 Å, which is accessible to chloride anions and water molecules and shows a good adsorption performance on dyes and I2 ethanol solutions.


 Please wait while we load your content...
Please wait while we load your content...