A metal–organic framework with tunable exposed facets as a high-affinity artificial receptor for enzyme inhibition†
Abstract
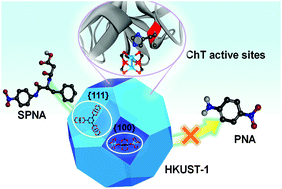
A copper-based metal–organic framework (MOF) HKUST-1 [Cu3(BTC)2] (H3BTC = 1,3,5-benzenetricarbocylic acid) was utilized as an artificial receptor to recognize a serine protease, α-chymotrypsin (ChT), with high affinity by both electrostatic interactions and coordination interactions. The buffer molecule 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid (HEPPS) could bind with copper(II) centers and form a negatively charged surface, leading to an electrostatic interaction between the cations on the surface of ChT. Meanwhile, according to Hard Soft Acid Base (HSAB) theory, the borderline Lewis acidic copper(II) centers could coordinate with the intermediate basic imidazole of His-57 at the active sites of ChT, preventing the binding of the substrate. Moreover, through coordination control by adding additional modulators, the different exposed facets with different arrangements of metal clusters and organic linkers could be obtained. Because the enzyme has different interactions with the metal centers and the organic linkers, the affinity between HKUST-1 and ChT could be well tuned. When more {111} facets than {100} facets existed in HKUST-1, the material exhibited better inhibition efficiency towards ChT.


 Please wait while we load your content...
Please wait while we load your content...