Improving the photocatalytic hydrogen production of SrTiO3 by in situ loading ethylene glycol as a co-catalyst†
Abstract
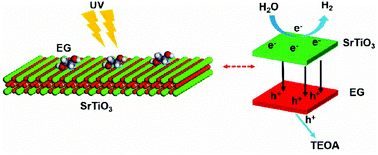
Co-catalysts such as precious metals are usually necessary to promote the photocatalytic generation of hydrogen from water through inhibiting the recombination of photo-generated carriers. However, precious metals are expensive and harmful to the environment. In this work, an ethylene glycol (EG) molecule, which was chemically anchored on SrTiO3 nanoparticles (EG@STO) via an in situ process, was used as a co-catalyst for the first time. The first-principles calculation shows that the EG molecule chemically anchored on SrTiO3 is beneficial for the directional migration of carriers and accelerates the hydrogen evolution reaction. A photocatalytic hydrogen generation efficiency of 237 μmol g−1 h−1 was achieved with EG@STO, which is 19.5 times higher compared to that of SrTiO3. Moreover, EG@STO remained stable after 32 hours of reaction; in other words, the EG molecule is designed as a new type of efficient and stable co-catalyst, which provides an alternative approach for better semiconductor photocatalytic hydrogen reduction.


 Please wait while we load your content...
Please wait while we load your content...