Remarkably improved oxygen evolution reaction activity of cobalt oxides by an Fe ion solution immersion process†
Abstract
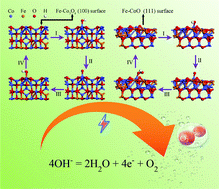
Cobalt oxides are economical and environmentally friendly electrocatalysts and they may be possible alternatives to noble metals for the oxygen evolution reaction. However, the most studied intrinsic cobalt oxides still need a high overpotential to overcome the current density threshold of oxygen evolution and are unstable for continuous reactions. Herein, we found that the catalytic activity of cobalt oxides improved significantly on soaking them in a weakly acidic iron–ion solution. The structure of the catalyst could be modified by the abovementioned treatment to reduce the overpotential, thus achieving improved catalytic efficiency for the oxygen evolution reaction. The formation of Fe-Co3O4 delivered a much lower overpotential of 280 mV at 10 mA cm−2 (with a small Tafel slope of 55 mV dec−1) compared with normal Co3O4 (409 mV) for the OER; additionally, Fe-CoO also exhibited a lower overpotential of 296 mV compared with normal CoO (361 mV). The activity of the Fe ion-treated cobalt oxides was promoted by a thousand CV scans, which might form an amorphous phase layer. Density functional theory (DFT) calculations also indicated that the theoretical overpotentials of Fe-Co3O4 and Fe-CoO were obviously lower than that of the untreated material. The treated cobalt oxides replaced cobalt atoms with a certain number of iron atoms, which dramatically changed the electronic structure and charge transfer rate.


 Please wait while we load your content...
Please wait while we load your content...