Synthesis of dual emitting iodocuprates: can solvents switch the reaction outcome?†
Abstract
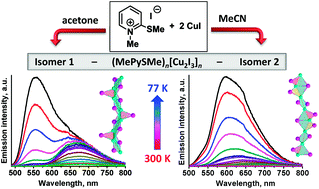
A remarkable effect of solvents on the reaction outcome has been discovered in the synthesis of halocuprates, as exemplified by the reaction of CuI with 1-methyl-2-methylthiopyridinium iodide, (Me–PySMe)I. When performed in acetonitrile, the reaction results in the selective self-assembly of iodocuprate (Me–PySMe)n[Cu2I3]n (1) featuring 1D double-stranded [Cu2I3]n ribbons. Surprisingly, upon exploiting acetone as a medium under other conditions being similar, isomeric 1D iodocuprate 2 containing one-stranded [Cu2I3]n chains is quantitatively formed. Iodocuprate 1 displays outstanding dual luminescence expressed by two emission bands at λmax = 680 and 550 nm, probably originating from the excited states of the cluster-centered (3CC) type and iodocuprate-to-cation charge transfer one, respectively. The resulting emission color of 1, being defined by the ratio of these bands, reversibly varies as a function of temperature spanning from deep-red (300 K) to yellow (77 K). Isomer 2, being also dual emissive, does not exhibit prominent thermochromic luminescence.


 Please wait while we load your content...
Please wait while we load your content...