Improving catalysis for electrochemical water splitting using a phosphosulphide surface†
Abstract
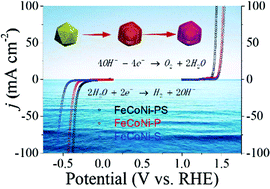
As an energy carrier, hydrogen shows great promise superior to the traditional fossil fuels to meet the ever-increasing global energy demands. Electrochemical water splitting has been considered to be an effective method to produce hydrogen cleanly. The two reactions, the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), in water splitting need efficient and Earth-abundant catalysts. Previous research indicates that the introduction of heteroatoms is an efficient way to improve the catalytic performance of metal sulfides due to the synergistic effect. Here, we demonstrate a yolk–shell phosphosulphide (FeCoNi-PS) with a porous structure, which shows good catalysis for both the HER and OER in alkaline media. In particular, the present catalyst showed geometrical current densities of 10 mA cm−2 and 100 mA cm−2 for the OER at overpotentials of 162 mV and 205 mV, respectively. In addition, the catalyst showed outstanding stability in a long-term operation test. This achievement sheds light on an effective route to design a high-performance, low-cost catalyst for large-scale water splitting.


 Please wait while we load your content...
Please wait while we load your content...