Highly efficient water splitting over a RuO2/F-doped graphene electrocatalyst with ultra-low ruthenium content†
Abstract
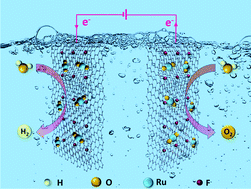
Poor durability and relatively high price are the main obstacles for RuO2 to be a good catalyst for overall water splitting. Here, RuO2 nanoparticles on fluorine-doped graphene (F-graphene) are synthesized with silicon nanowires serving as a sacrificial template. Small RuO2 nanoparticles are homogeneously distributed on the surface of graphene without aggregation due to the suitable reducibility of Si–H. The content of Ru in the RuO2/F-graphene electrocatalyst is only 6.9 wt%, but it can exhibit better HER or OER performance and stability than 20% Pt/C or commercial RuO2. The potentials @ 10 mA cm−2 of RuO2/F-graphene for overall water splitting in 1 M KOH and 1 M PBS solution are only 1.56 and 1.73 V respectively, lower than those of commercial RuO2 (1.72 and 2.34 V). Furthermore, the addition of fluorine with strong electronegativity can enhance the electrical conductivity and stability of the RuO2/F-graphene electrocatalyst. The decreased noble metal content and increased stability of RuO2/F-graphene catalysts may have practical applications.


 Please wait while we load your content...
Please wait while we load your content...