Ultrafine Rh nanoparticles confined by nitrogen-rich covalent organic frameworks for methanolysis of ammonia borane†
Abstract
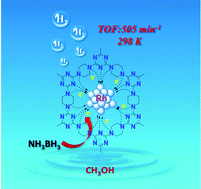
Nitrogen-rich covalent organic frameworks (COFs) with a high content of nitrogen and an inherent porous skeleton are considered as a remarkable support for encapsulating active metal nanoparticles (MNPs). Herein, a cost-effective and nitrogen-rich COF (PC-COF) synthesized from piperazine and cyanuric chloride was used as a carrier for encapsulating Rh NPs via a metal–nitrogen (M–N) coordination reduction strategy. Numerous precisely tailored N atoms within the PC-COF facilitate the construction of Rh–N complexes between Rh ions and N atoms, which can be in situ reduced to produce ultrafine Rh NPs confined in the pores of the PC-COF. The optimized Rh/PC-COF catalyst shows uniform dispersibility and a fairly narrow distribution (1.4–2.6 nm) of Rh NPs, giving an ultrahigh catalytic activity for the methanolysis of ammonia borane (AB) with a total turnover frequency (TOF) of 505 min−1 at 298 K. The excellent catalytic performances can be ascribed to the ultrafine particle size, electronic-rich Rh NPs, and the synergistic effect of the Rh NPs and PC-COF. The M–N coordination reduction strategy for encapsulating ultrafine MNPs into the pores of COFs opens a valuable pathway for developing metal nanocatalysts with high catalytic performance and excellent stability.


 Please wait while we load your content...
Please wait while we load your content...