Bifunctional PtCu electrocatalysts for the N2 reduction reaction under ambient conditions and methanol oxidation†
Abstract
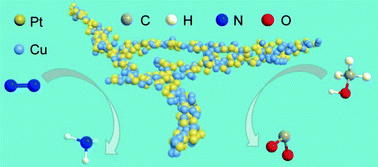
In our work, we demonstrated network PtCu nanocrystals, in which the tuneable composition, clean surface, and ultrasmall size (diameter < 5 nm) endow them with excellent electrocatalytic performances in both N2 reduction reaction under ambient conditions and methanol oxidation. A detailed investigation suggests that their electrocatalytic activity is highly composition dependent. We confirmed the production of NH3 from N2 through a systematic study. The optimum Pt6Cu nanoalloys exhibit 2.3-fold and 20.6-fold increase in reduction activity toward N2 compared with Pt and Cu nanocrystals. Their faradaic efficiency value was also highly improved. Moreover, these bifunctional Pt6Cu nanostructures also show excellent catalytic properties during methanol electrooxidation. The specific activity toward methanol oxidation reaches up to 21.3 mA cm−2, which is 3.6 times that of commercial Pt/C catalysts. This work thus provides a promising approach to design the alloy composition for bi- or multi-functional applications.


 Please wait while we load your content...
Please wait while we load your content...