Ligand-centered reactivity of a pseudo-dearomatized phosphorus-nitrogen PN3P* rhodium complex towards molecular oxygen at room temperature†
Abstract
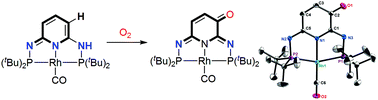
The ligand of an organometallic complex is typically considered as a spectator which can be modified to tune the steric and electronic properties of the coordination environment. We herein demonstarted a PN3P*Rh-CO pincer system where one of the C–H bonds of the pseudo-dearomatized pyridine ring was oxidized by O2 at room temperature to create an α,β-unsaturated carbonyl functionality on the ligand backbone. The resulting metal complex with the post-modified PN3P ligand readily reacts with thiophenol and 4-methylaniline to afford the corresponding oxidative Michael addition products.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...