Metal–ligand cooperativity of a Co–P moiety†
Abstract
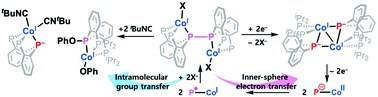
A novel cobalt system featuring a Co–P moiety has been synthesized to study its metal–ligand cooperativity. A reversible conversion of a phosphide group to a P–P bond found in a dimeric cobalt(I) complex involves electron transfer between Co and P, in which a single electron transfer is successfully coupled with a redox change in each cobalt ion. Upon coordination of a π-acidic ligand such as acetonitrile and isocyanide at the cobalt site, the transformation of a dimeric to a monomeric cobalt complex occurs, which involves the migration of a phenolate group. During the conversion, a P–P bond was cleaved and converted to phosphide and phosphinite. Current Co–P metal–ligand cooperativity presented in this work has been explored structurally, spectroscopically and theoretically.


 Please wait while we load your content...
Please wait while we load your content...