Regulating the structural dimensionality and dynamic properties of a porous dysprosium coordination polymer through solvent molecules†
Abstract
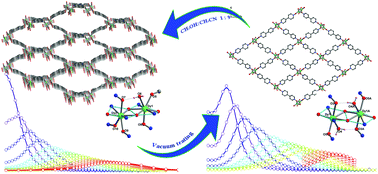
A novel three-dimensional porous dysprosium coordination polymer {[Dy2L2(bpdo)2(H2O)(CH3OH)]·2ClO4·2CH3CN}n (1) has been successfully assembled by combining a superparamagnetic building block of [DyLCl(CH3OH)]2 with the organic ligand 4,4′-bipyridine-N,N′-dioxide (bpdo) (H2L = N′-(2-hydroxybenzylidene)picolinohydrazide). Interestingly, upon the release and/or absorption of different solvent molecules, 1 undergoes reversible single-crystal-to-single-crystal (SCSC) transitions, resulting in the formation of another layer compound of {[DyL(bpdo)(H2O)]·ClO4·2H2O}n (2). This structural transformation involves the breaking and reforming of coordination bonds and leads to significant changes in the relaxation behaviour expected for these coordination polymers, as evidenced by the increase in effective energy barriers from 133 K (1) to 264 K (2) and the slowdown of quantum tunnelling at low temperature. The theoretical calculations indicate that the distinct relaxation behaviours arise mainly from the variation in the coordination geometry around the dysprosium ions. Our work shows that guest molecules can reversibly induce remarkable structural changes in a porous dysprosium coordination polymer and significantly affect its dynamic properties.


 Please wait while we load your content...
Please wait while we load your content...