A Ni/Fe-based heterometallic phthalocyanine conjugated polymer for the oxygen evolution reaction†
Abstract
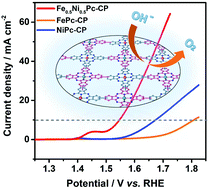
Ni/Fe-Based compounds have been demonstrated as efficient and economical oxygen evolution reaction (OER) electrocatalysts. However, the intrinsic reaction mechanism of the Ni/Fe-based compounds still remains unclear and under debate. Herein a Ni/Fe-based heterometallic compound Fe0.5Ni0.5Pc-CP has been prepared by a flexible method through coupling of Ni[Pc(ethynyl)4] and Fe[Pc(I)4]. The as-prepared Fe0.5Ni0.5Pc-CP exhibits an excellent performance toward the OER with the overpotential at 10 mA cm−2 as low as 317 mV, superior to those of FePc-CP (570 mV) and NiPc-CP (440 mV) and even comparable to that of commercial RuO2 (320 mV). Importantly, both the experimental and theoretical methods demonstrate that the improved OER performance of Fe0.5Ni0.5Pc-CP relative to the homometallic analogues can be attributed to the electronic interactions between the adjacent Fe and Ni atoms in Fe0.5Ni0.5Pc-CP, which facilitate the generation of the high-valence Fe/Ni species in Fe0.5Ni0.5Pc-CP and in turn boost the OER. The present result helps gain insight into the reaction mechanism of Ni/Fe-based OER electrocatalysts and further develop OER electrocatalysts with promising performance.


 Please wait while we load your content...
Please wait while we load your content...