Interaction of U(vi) with α-MnO2@layered double hydroxides by combined batch experiments and spectroscopy studies†
Abstract
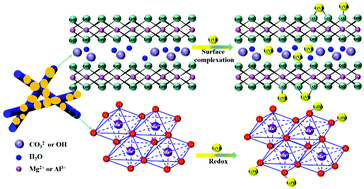
Uranium is of high concern in the field of environmental remediation because of its high fluidity, radioactivity, biological toxicity and long life. Removing U(VI) from wastewater is of great significance to both environment and biology. Herein, the composite adsorbent α-MnO2@LDHs composed of α-MnO2 and layered double hydroxides (LDHs) was constructed, and U(VI) adsorption experiments under different conditions were systematically carried out. The results manifested that the maximum U(VI) removal capacity of α-MnO2@LDHs was 135.52 mg g−1 at 298 K through the formation of inner-sphere surface complexes and redox reactions. In particular, at 328 K, the removal amount reached 564.97 mg g−1, which suggested the potential to treat high-temperature radioactive wastewater. Furthermore, α-MnO2@LDHs exhibited stability in a wide range of ionic strength (0.001–0.1 M) and pH (5.0–12.0), strong resistance to foreign ion interference, and rapid adsorption capacity. These made α-MnO2@LDHs an outstanding candidate for repair materials, and performed well even in simulated environments. In-depth and systematic spectra analysis revealed that the active functional groups were Al– and Mg–OH. Mn3+ and CO32− also made important contributions to the combination of U(VI). This work might promote the development of MnO2 combined with various metal LDHs, providing a reference for designing excellent repair materials.


 Please wait while we load your content...
Please wait while we load your content...