Light-induced disruption of an acyl hydrazone link as a novel strategy for drug release and activation: isoniazid as a proof-of-concept case†
Abstract
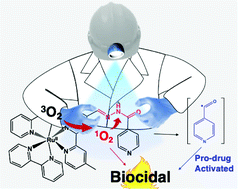
Aldehydes and acyl hydrazines have been employed in a conjugation reaction that produces an acyl hydrazone bridge. A drawback to this linkage is hydrolysis, which prevents its broader use in a controllable manner. We have developed a new strategy for the cleavage of this group using a ruthenium(II) metal complex as a photogenerator of singlet oxygen. Isoniazid, an anti-tuberculosis prodrug, was chosen as a proof-of-concept compound; in this case it was coupled to an aldehyde-derivative trisbipyridine ruthenium(II) metal complex. Studies carried out using HPLC, MS, EPR, fluorescence and UV-vis showed that light could efficiently disrupt the acyl hydrazone linkage with the production of an isonicotinoyl radical. In addition, biological studies showed that bacterial strains and cancer cells became susceptible upon light irradiation. These results support a novel strategy of photoactivation based on a ruthenium metal complex conjugated to a pro-drug through an acyl hydrazone linkage, opening a broad range of applications.


 Please wait while we load your content...
Please wait while we load your content...