Recent advances in the mechanisms of the hydrogen evolution reaction by non-innocent sulfur-coordinating metal complexes
Abstract
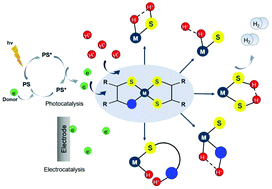
Efficient hydrogen generation from aqueous protons through direct conversion of solar energy is an essential prerequisite of a future hydrogen economy and easily accessible renewable energy. For achieving this goal, the effective synthesis of a catalyst that can promote the hydrogen evolution reaction (HER) is in demand. Several mononuclear non-noble metal complexes carrying non-innocent S donating ligands have been reported as efficient photocatalysts and electrocatalysts for proton reduction. A thorough understanding of the elementary steps of hydrogen formation allows one to derive structure–property relationships that can guide future catalyst design. In this review, we highlight the mechanisms of the homogeneous HER for these catalysts in the light of photocatalytic, electrocatalytic and computational data with the main concern of elucidating the interplay between the localization of the electron density and the hydrogen evolution reaction pathway. In addition, the effects of π-conjugation, heteroatoms and electron accepting/donating moieties on the basicity of the metal center and catalyst overpotential are discussed to rationalize the formation of a metal or ligand hydrid intermediate. Moreover, some suggestions are provided for future use in the design of effective HER catalysts.
- This article is part of the themed collection: 2020 Inorganic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...