Structural, electrochemical and photophysical behavior of Ru(ii) complexes with large bite angle sulfur-bridged terpyridyl ligands†
Abstract
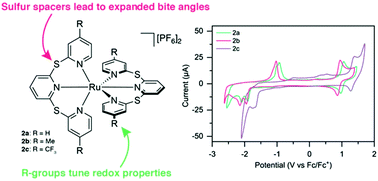
Ruthenium(II) terpyridyl species typically suffer from a distortion away from the ideal octahedral geometry due to the rigidity of the terpyridine ligand. In this study we report a series of new sulfur-bridged terpyridyl ligands (1a–c) which offer enhanced flexibility due to sulfur spacers linking the pyridine rings, in addition to the ability to tune electronic properties with electron-donating (Me) and electron-withdrawing (CF3) substituents in the 4 and 4′′-positions of the sulfur-bridged ligands. The resulting Ru(II) complexes are detailed herein as both homoleptic (2a–c) and heteroleptic (3a–c) species, with the synthesis, structural characterization, photophysical and redox properties discussed, and the sulfur-bridged ligands allowing geometries closer to that of a “perfect” octahedron. All six species were found to be non-emissive at room temperature (with emissivity increasing on cooling to 77 K), and the electrochemical properties were greatly altered due to the increased bite angle of the sulfur-bridged ligands. The electrochemical and photophysical HOMO–LUMO gap can be easily tuned by altering the substituents in the 4 and 4′′-positions.


 Please wait while we load your content...
Please wait while we load your content...