Chemo-enzymatic synthesis of a levoglucosenone-derived bi-functional monomer and its ring-opening metathesis polymerization in the green solvent Cyrene™†
Abstract
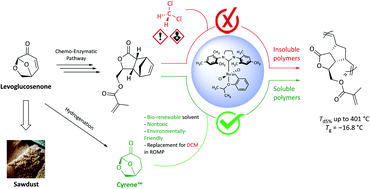
The quite recent, yet quickly expanding, norbornene-levoglucosenone-based monomer family was extended to include a novel bi-functional methacrylate monomer that can be readily synthesized through a chemo-enzymatic pathway. The norbornene moiety was selectively polymerized via ring-opening metathesis polymerization (ROMP) under ambient conditions where Cyrene™ was explored for the first time as a green bio-alternative organic solvent for ROMP reactions. The activity of the metathesis catalysts was finely tuned in Cyrene™, versus common and toxic solvents such as dichloromethane, resulting in highly thermostable functional polymers with a Td5% up to 401 °C and a Tg of −16.8 °C.
- This article is part of the themed collection: Sustainable Polymers


 Please wait while we load your content...
Please wait while we load your content...