Hyperbranched polymers with step-growth chemistries from transfer-dominated branching radical telomerisation (TBRT) of divinyl monomers†
Abstract
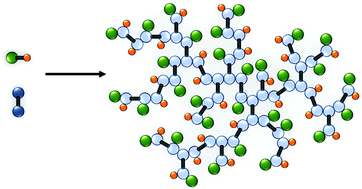
The commercial synthesis of polymers is generally limited to two main mechanisms that are typically considered to be mutually exclusive, namely step-growth and chain-growth polymerisation. This also defines the vast number of academic advances in macromolecular synthesis including the increasingly studied reversible deactivation radical polymerisation techniques (chain-growth) and complex polymer architectures such as dendrimers (step-growth). We report here a new synthetic strategy that utilises conventional free radical chain-growth chemistry, under modified telomerisation conditions, to form branched polymers containing chemistries conventionally formed under step-growth conditions. Telomerisation is typically limited to small molecule synthesis and employs addition across the unsaturated bond of a substrate, whilst minimising intermolecular reaction between substrates. Through the careful manipulation of reaction conditions, we have created a ‘transfer dominated branching radical telomerisation’ mechanism that creates branched polymers containing step-growth motifs from multi-vinyl monomers, with molecular weights in excess of 1000 kg mol−1, using industrially relevant free radical chain-growth chemistry. The scope of this approach is considerable, allowing access to entirely new macromolecular structures.


 Please wait while we load your content...
Please wait while we load your content...