An ε-caprolactone-derived 2-oxazoline inimer for the synthesis of graft copolymers†
Abstract
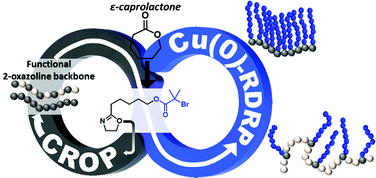
An inimer approach is presented here for the formation of bottlebrush copolymers consisting of a poly(2-oxazoline) backbone and acrylate branches. A hydroxyl group containing 2-oxazoline (2-n-pentanol-2-oxazoline) was synthesized from ε-caprolactone, which is a sustainable starting material. 2-n-Pentanol-2-oxazoline was then further functionalized with a bromoisobutyrate group, which was used to initiate a Cu(0)-mediated reversible-deactivation radical polymerisation (RDRP) of acrylates. This compound is termed an “inimer” because it contains both a monomer (2-oxazoline) and an initiator (the RDRP initiator in this study). Herein, we report the use of an inimer to form 2-oxazoline-based polymer backbones through cationic ring opening polymerisation, and further polymerisation via the Cu(0)-mediated RDRP of acrylates was achieved by initiating from the RDRP-initiator sites embedded within the poly(2-oxazoline) backbone. Furthermore, statistical copolymers of 2-ethyl 2-oxazoline and the inimer were also formed leading to statistically distributed radical initiating sites, which were then used to form graft copolymers of varying densities.


 Please wait while we load your content...
Please wait while we load your content...