Insight into the synthesis of N-methylated polypeptides†
Abstract
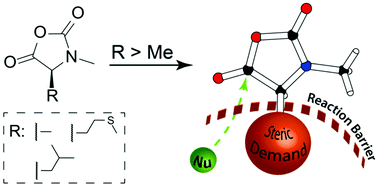
The ring-opening polymerization (ROP) of N-carboxy anhydrides (NCAs) is mostly divided into two classes: NCAs of α-substituted amino acids and N-methylated NCAs of α-unsubstituted glycine derivatives (NNCAs). The use of both monomer types offers different mechanistic features and results in a multitude of functional materials. To combine these properties, the synthesis and ROP of α-substituted and N-methylated NCAs (αNNCAs) of several amino acids were investigated. The current study provides insight into the influence of polymerization conditions and the limitations caused by the enhanced steric demand of the amino acid NCA monomers and their N-methylated derivatives. Namely, the effects of solvent polarity (DMF and DCM) and steric demand of the initiator by using neopentyl amine (NPA) and n-butyl amine (nBu) were studied. Analysis by HFIP-GPC and MALDI-ToF MS reveals that the polymerization and the resulting polymers are tremendously affected by the steric demand of both the initiators and the monomers, while electronic effects seem to have only minor influences. The experimental results are further compared with computational studies, based on coupled cluster (CC) calculations, which underline that electronic effects are of lower importance than steric constraints for the ROP of αNNCAs. Moreover, poly(N-methyl-L-methionine) forms helical secondary structures in solution. Therefore, this work combines mechanistic studies of the ROP of αNNCAs with initial studies on the solution properties of these polypeptides.
- This article is part of the themed collection: Polymer Chemistry Pioneering Investigators 2021


 Please wait while we load your content...
Please wait while we load your content...