Metal-free anionic polymerization of n-hexyl isocyanate catalyzed by phosphazene bases†
Abstract
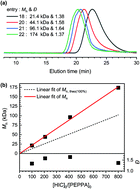
Metal-free anionic polymerization of n-hexyl isocyanate (HIC) catalyzed by phosphazene bases in THF at −98 °C under 10−6 Torr was attempted to obtain poly(n-hexyl isocyanate) (PHIC) peptide mimics with a high purity. tert-Butylimino-tris(dimethylamino)phosphorene (t-BuP1), 1-tert-butyl-2,2,4,4,4-pentakis(dimethylamino)-2λ5,4λ5-catenadi(phosphazene) (t-BuP2), and 1-tert-butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)-phosphoranylidenamino]-2λ5,4λ5-catenadi(phosphazene) (t-BuP4) with the characteristic orders of basicity and bulkiness (t-BuP1 < t-BuP2 < t-BuP4) were used as catalysts to activate N-phenethyl-3-phenylpropanamide ((PhEt)2OCNH, PEPPA). Only t-BuP2 and t-BuP4 generated the initiating amidate anions paired with each phosphazenium cation, which produced PHICs with MWs higher than the theoretical ones and characteristic ranges of dispersities. A moderately low dispersity was achieved by t-BuP2. The anionic polymerization of HIC catalyzed by t-BuP2 with molar feed ratios of the monomer to the initiator ([HIC]0/[PEPPA]0) within the range 207–801 produced PHICs with high yields (96.9–98.7%) and high MWs (Mn = 44.1–174 kDa, Đ = 1.37–1.64) that were linearly controlled by the feed ratios, even though no polymers were obtained by t-BuP1, and the products whose production was catalyzed by t-BuP4 had unpredictable MWs and high dispersities (Đ = 1.82–2.20).


 Please wait while we load your content...
Please wait while we load your content...