Direct fluorination as a one-step ATRP initiator immobilization for convenient surface grafting of phenyl ring-containing substrates†
Abstract
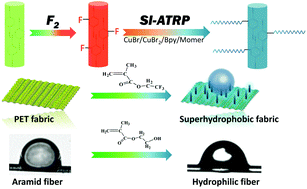
Generally, surface initiated-atom transfer radical polymerization (SI-ATRP) often suffers from the immobilization of the initiator on the surface-inert substrate. Developing an effective and versatile one-step method to anchor the priming sites while retaining the bulk properties of the substrate still faces challenges. In this work, direct fluorination is proposed to treat the benzene ring-containing substrate and the C–F introduced on the benzene ring can be used as an initiation site to achieve SI-ATRP. Computer and molecular simulation experiments have shown that the C–F added on the phenyl ring is more suitable for initiating ATRP than the C–F on the alkane chain. Thereafter, polyethylene terephthalate (PET) fabric was successfully grafted with fluorinated methacrylate polymer via direct fluorination-initiated ATRP, resulting in durable superhydrophobic properties, which demonstrated the practicability of the method. Furthermore, fluorination-initiated ATRP presents universality with other phenyl ring-containing polymeric substrates, such as aramid fiber, polyimide fiber and poly-p-phenylene benzobisoxazole fiber. Among them, the fluorinated aramid fiber can be grafted with hydroxyethyl methacrylate to obtain increased interfacial shear strength.


 Please wait while we load your content...
Please wait while we load your content...