Balancing the transesterification reactivity of isosorbide with diphenyl carbonate: preferential activation of exo-OH†
Abstract
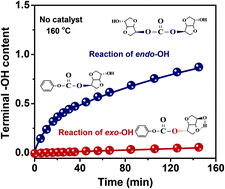
The exo-hydroxyl group (exo-OH) on isosorbide (ISB) has long been asserted as a highly reactive moiety compared with the endo-hydroxyl group (endo-OH). In this study, calculations based on density functional theory and experiments without adding catalysts reveal that endo-OH has strong nucleophilic ability, and in the case of transesterification with diphenyl carbonate, the nucleophilic attack surmounts steric hindrance in rendering endo-OH more reactive than exo-OH. The kinetics of transesterification with different catalysts is investigated to determine the catalytic reactivity and molecular structure evolution. The results show that preferential activation of exo-OH moieties can be achieved either by reducing the coordination ability of catalytic cations (lg β1) or by enhancing the alkalinity of catalytic anions. Despite the low reactivity of exo-OH on ISB monomers, terminal exo-OH on carbonate oligomers exhibits higher reactivity than terminal endo-OH, justifying the experimental fact that ISB-based polycarbonates (ISB-PCs) are mostly terminated by endo-OH. Balancing the reactivity between endo-OH and exo-OH can be promoted by increasing the reaction temperature, thus accelerating transesterification to a high equilibrium constant. These findings help to clarify the mechanism of ISB transesterification and provide new strategies for the synthesis of high-molecular-weight ISB-PCs.


 Please wait while we load your content...
Please wait while we load your content...