Synthesis of polymeric micelles with dual-functional sheddable PEG stealth for enhanced tumor-targeted drug delivery†
Abstract
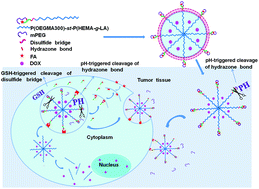
Conjugation of folic acid (FA) onto the surface of nanocarriers for active targeting is the mainstream strategy to improve the targeting efficiency of delivery vehicles for enhanced anticancer drug delivery; however, this strategy suffers from the fast clearance of nanocarriers caused by an immunological response and the nonspecific cellular uptake of nanocarriers compromising therapeutic efficiency and causing serious side effects. To address this dilemma, a dual-functional sheddable monomethoxypolyethylene glycol (mPEG) stealth component for both protection of the FA targeting ligand and extracellular stabilization of the micelles was incorporated into the polymeric micelles to realize simultaneous tumor-triggered targeting and intracellular micelle destabilization for enhanced cellular uptake and drug release. Specifically, the mPEG stealth component containing a tumor acidic pH-cleavable hydrazone bond was linked to an FA-conjugated amphiphilic block-statistical copolymer, FA-poly(oligo(ethylene glycol)monomethyl ether methacrylate)-st-poly(2-hydroxyethyl methacrylate-g-lactide) (P(OEGMA300)-st-P(HEMA-g-LA)) via highly efficient click coupling. The deshielding of the mPEG stealth component at the weakly acidic pH of the tumor site can expose the FA targeting ligand that is shielded under physiological conditions for active targeting, and trigger the destabilization of the self-assembled micelles for accelerated drug release. Meanwhile, reducibly conjugating the FA targeting ligand via a disulfide bond can further promote the targeting efficiency of the micelles by continuous folate receptor (FR)-mediated endocytosis. A panel of block-statistical copolymers with three different hydrophilic weight fractions was synthesized to investigate the structure–property relationship of this micelle construct. The optimized micelle formulation based on mPEG(FA)-P(OEGMA300)4-st-P(HEMA-g-LA)4 is relatively stable at pH 7.4 with a hydrodynamic diameter (Dh) of 41 nm, and the intracellular simultaneous cleavage of the disulfide links and hydrazone bonds led to significantly promoted destabilization of the drug-loaded micelles for promoted drug release. Therefore, the adoption of a sheddable mPEG stealth component with dual functionalities for greater cellular uptake and faster drug release of micelles provides a powerful means of enhanced anticancer drug delivery.


 Please wait while we load your content...
Please wait while we load your content...