Nitroxide polymer gels for recyclable catalytic oxidation of primary alcohols to aldehydes†
Abstract
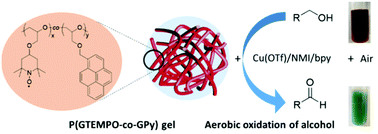
Nitroxide radicals have great applications in energy storage, catalysis, and biomedicine. The combination of nitroxide radicals and (bpy)copper is an efficient and green catalytic system that mimics galactose oxidase and uses oxygen as a primary oxidant. However, the separation and reuse of nitroxide radicals from the reaction have been challenging. Herein, we demonstrate the synthesis of a polyether-based gel with pendant 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) radicals and its application as a recyclable catalyst to oxidise primary alcohols to aldehydes in air. TEMPO-containing polyether gel is produced via anionic polymerization in a polar environment, driven by the hydrophobic π–π stacking of pyrene functionalities. The content of pyrene functional comonomers (i.e. 10 mol%), the polarity of polymerization solvents, and the chemical composition of the backbone are equally important for physical crosslinking and gel formation. This TEMPO-containing polyether gel showed the great capability of catalytically oxidising a variety of alcohols to aldehydes with (bpy)copper as a co-catalyst in air. The gel can be easily separated and recycled from the reaction for repeated catalysis without the leakage of TEMPO radicals.


 Please wait while we load your content...
Please wait while we load your content...