Synthesis of cyclic poly(2-ethyl-2-oxazoline) with a degradable disulfide bond†
Abstract
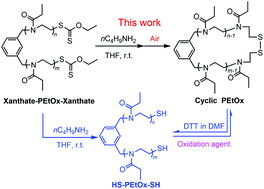
Cyclic poly(2-ethyl-2-oxazoline) (PEtOx) with a degradable disulfide bond has been synthesized by combining cationic ring opening polymerization (ROP) and reversible thiol/disulfide exchange chemistry. In greater detail, a living linear PEtOx with two cationic propagating terminals was initially synthesized using 1,3-bis(bromomethyl)benzene as a difunctional initiator. Both the living ends of PEtOx were then reacted with potassium ethyl xanthate to afford α,ω-xanthate end-functionalized PEtOx (xanthate-PEtOx-xanthate). The xanthate groups were then in situ cleaved using n-butylamine as a cleaving agent as well as a base in the same reaction media, which produced α,ω-thiol end-functionalized PEtOx (HS-PEtOx-SH). The cyclic polymer was eventually obtained by the successive intramolecular oxidation coupling of the thiol terminals of HS-PEtOx-SH to form a disulfide bond as a ring-closure link. For the polymeric information, 1H NMR spectroscopy, SEC spectroscopy, MALDI-TOF-MS, and UV-visible spectroscopy were combined to characterize the linear and cyclic polymers. Ellman's assay was used to determine the thiol content in the cyclic and thiol terminated polymers. Clean cyclic PEtOxs with number-average molar masses around 2000 (2 K) and 4000 (4 K) g mol−1 were obtained at an unreported high polymer concentration of 5 g L−1 (ca. 2.5 × 10−3 M for the 2 K polymer and ca. 1.25 × 10−3 M for the 4 K polymer) in THF. In addition, the synthetic route was compared with the intramolecular copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction and proven to be more advantageous in terms of reactant concentration, yield, purification, and bonding reversibility.


 Please wait while we load your content...
Please wait while we load your content...